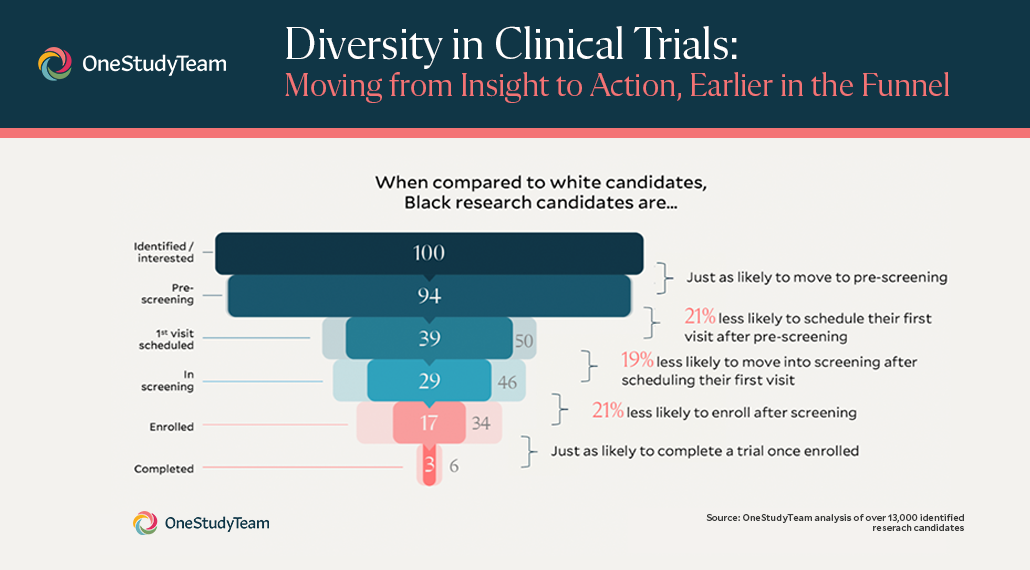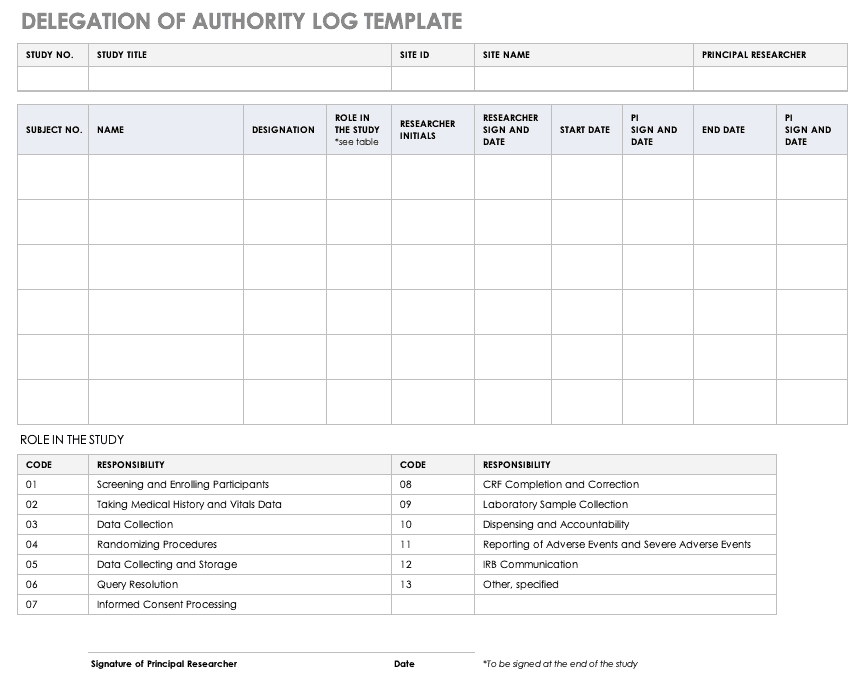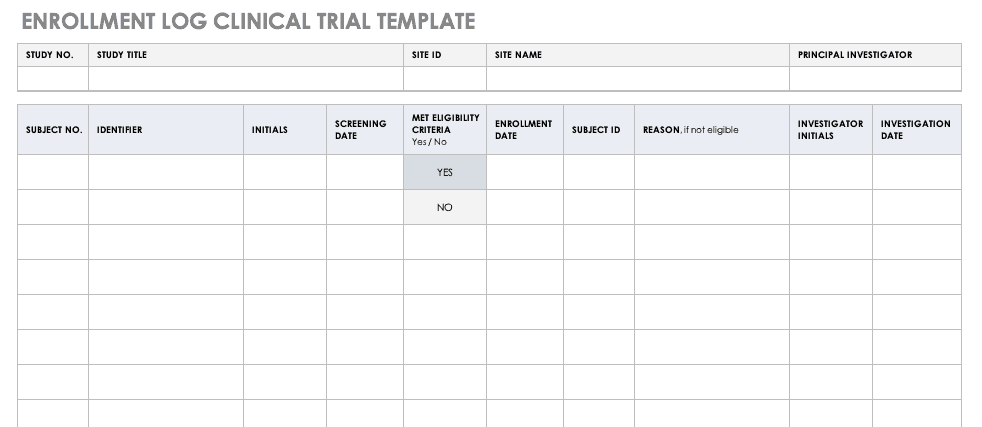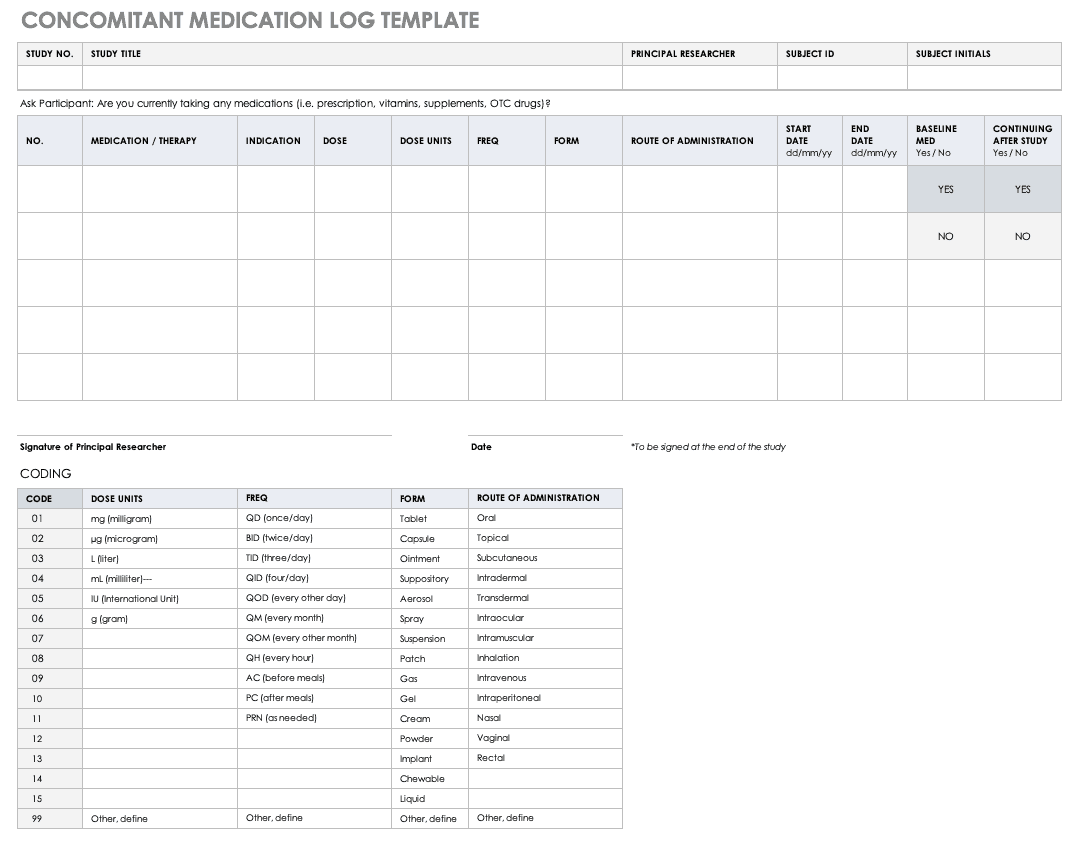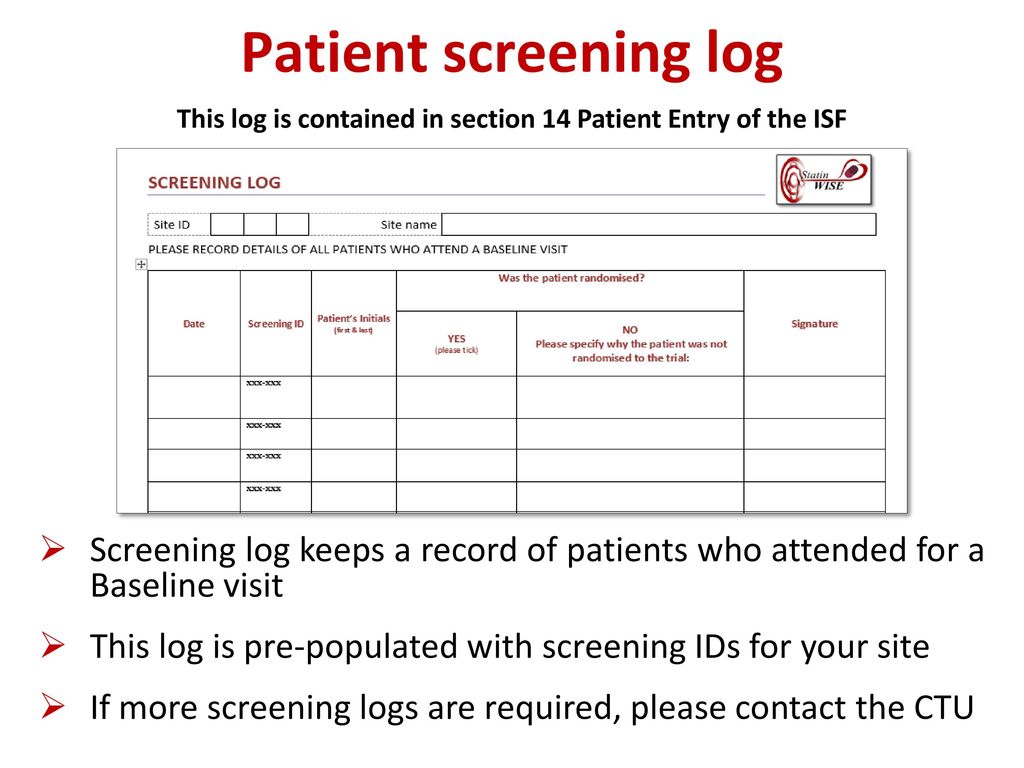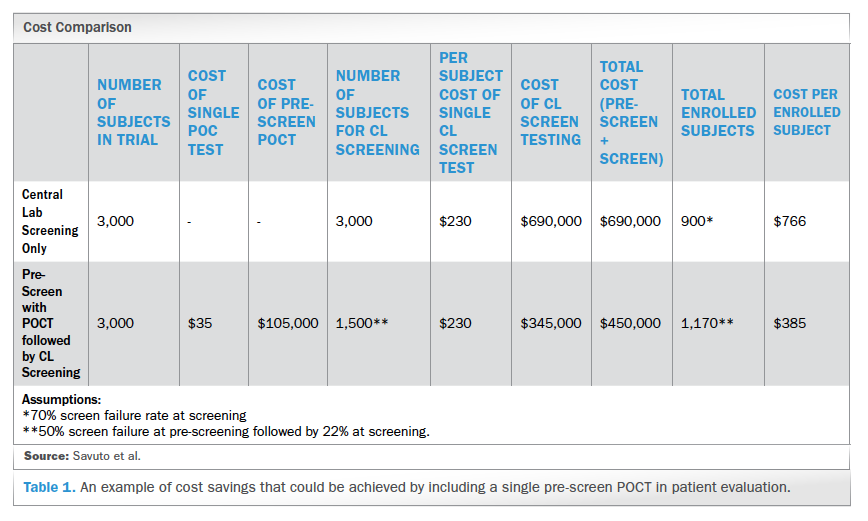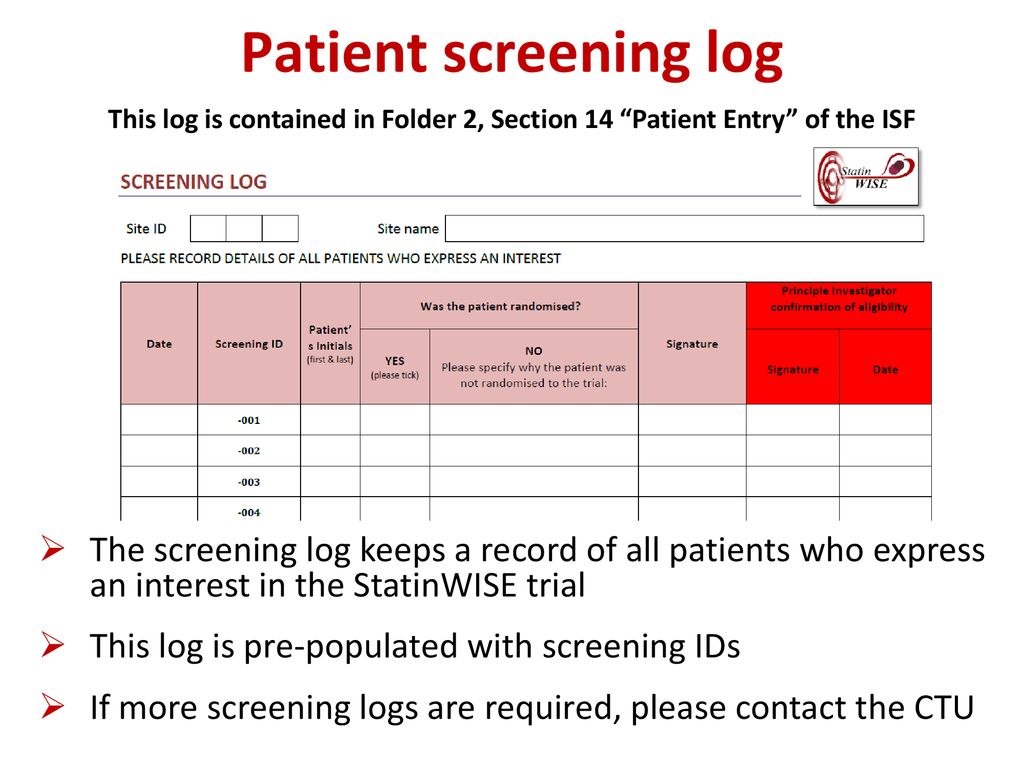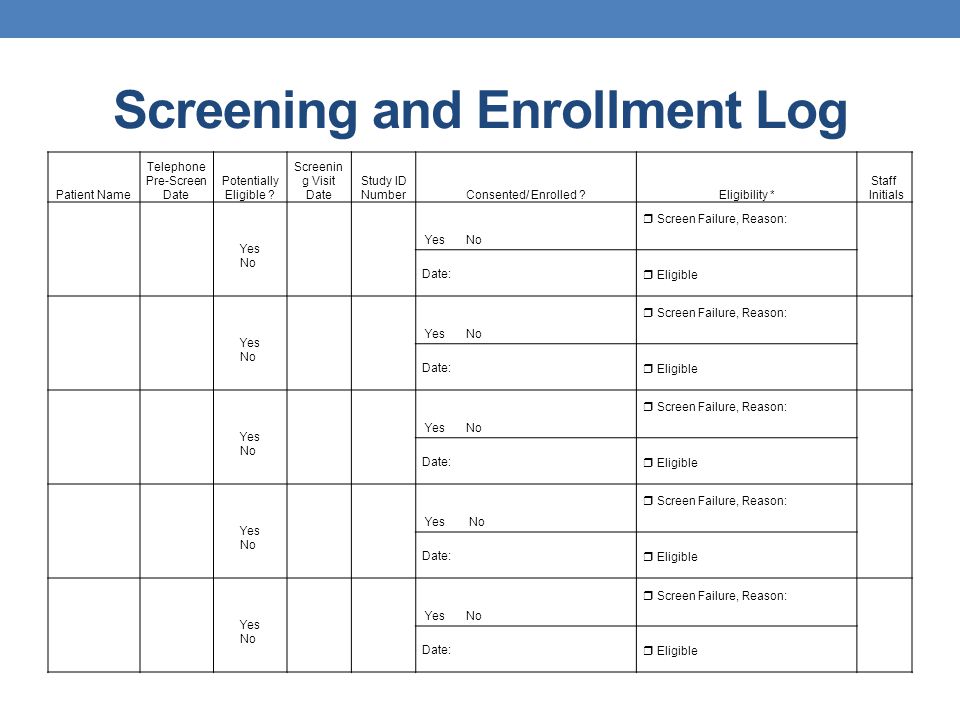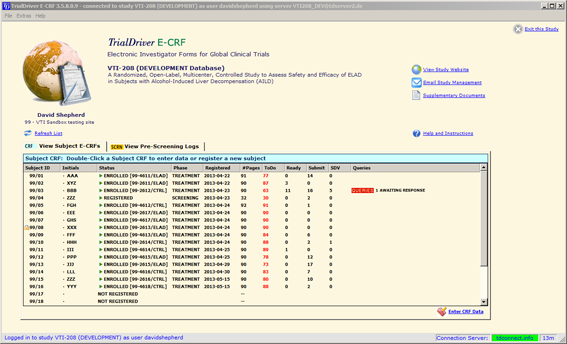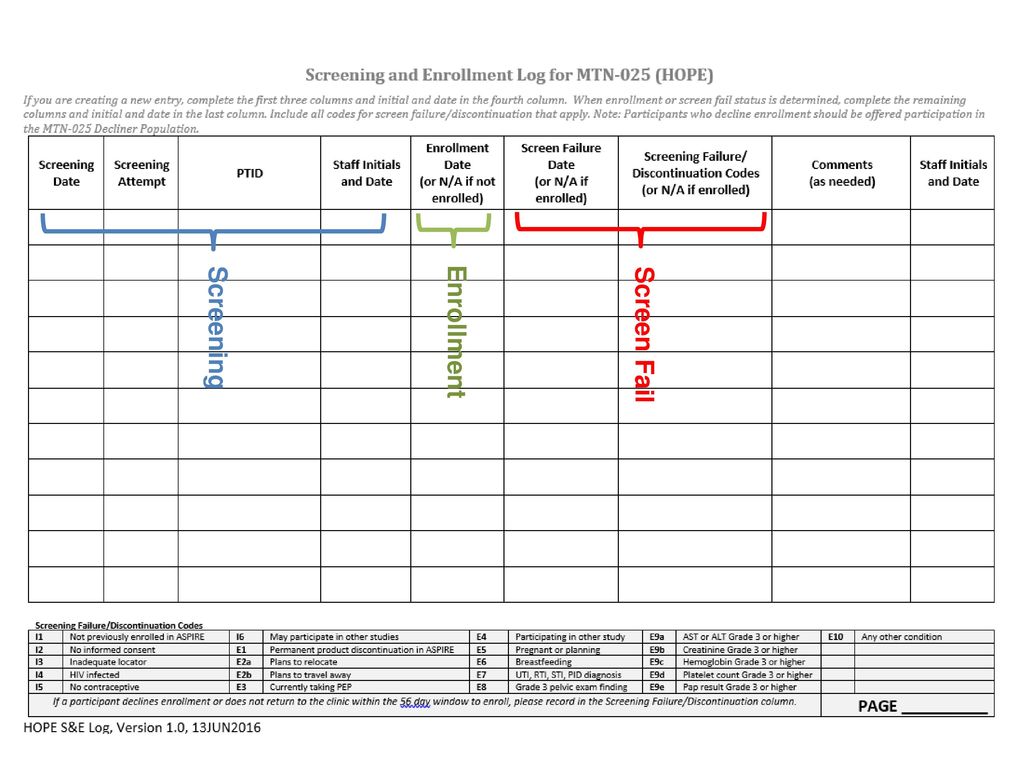
Overview of Screening Visit Procedures, PTID Assignment, Screening & Enrolment Logs, and Screen Failures Objectives: Review screening visit procedures. - ppt download

Recruitment and retention of the participants in clinical trials: Challenges and solutions | Semantic Scholar
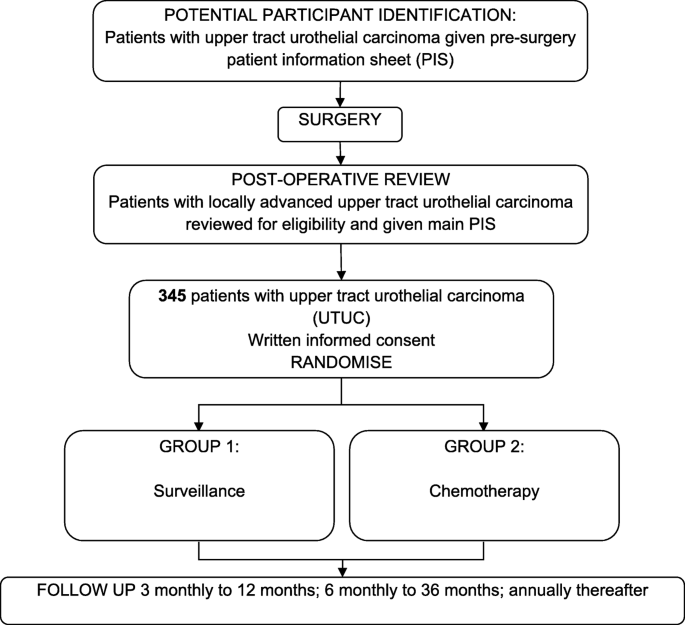
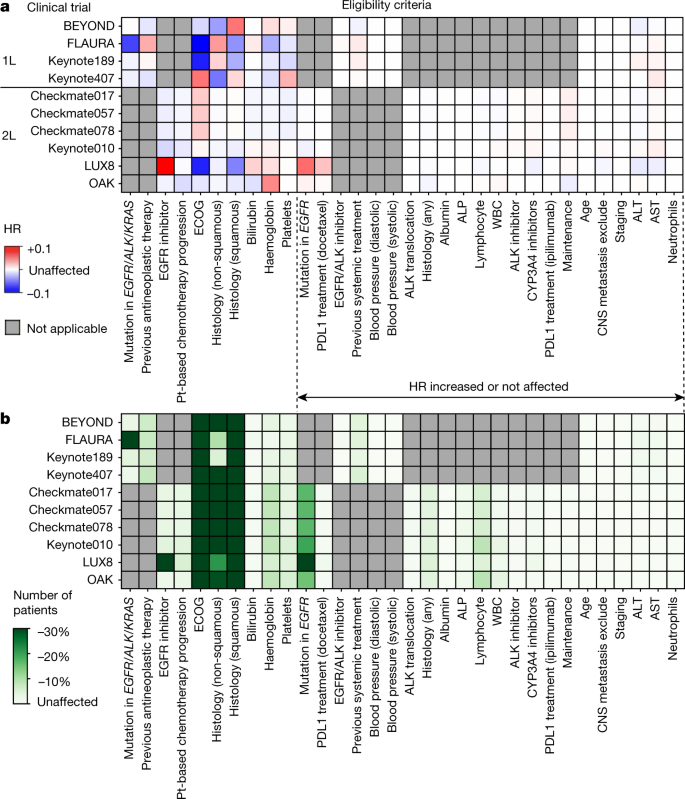
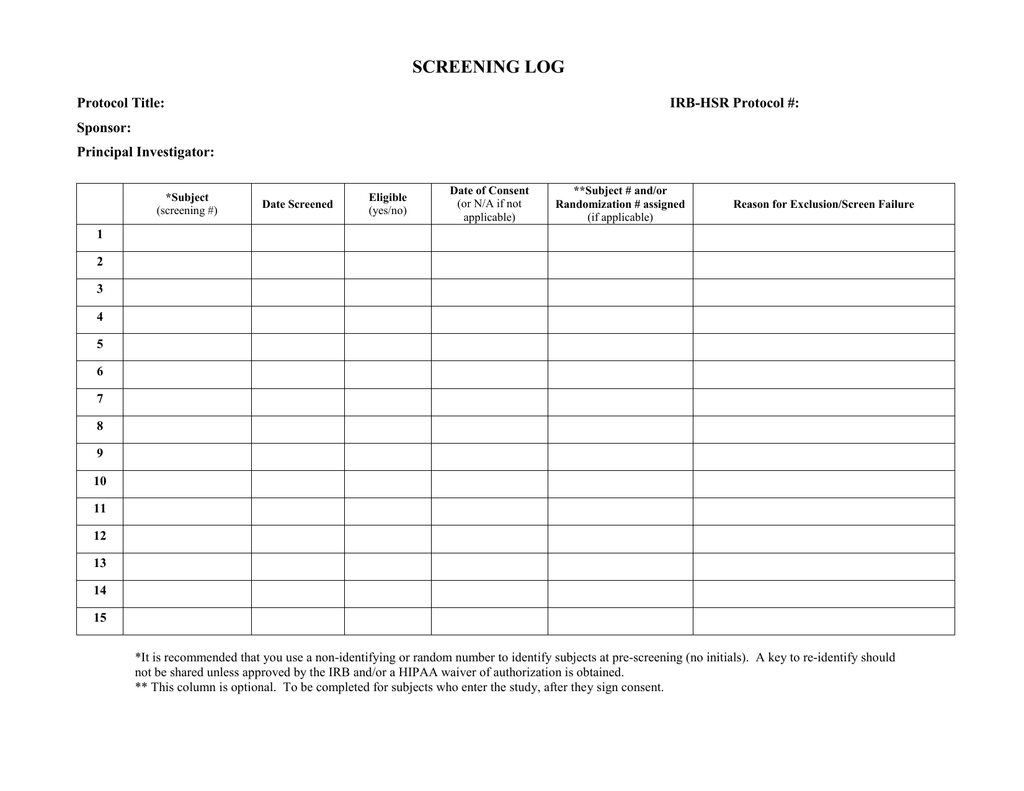
The implementation and utility of patient screening logs in a multicentre randomised controlled oncology trial | Trials | Full Text
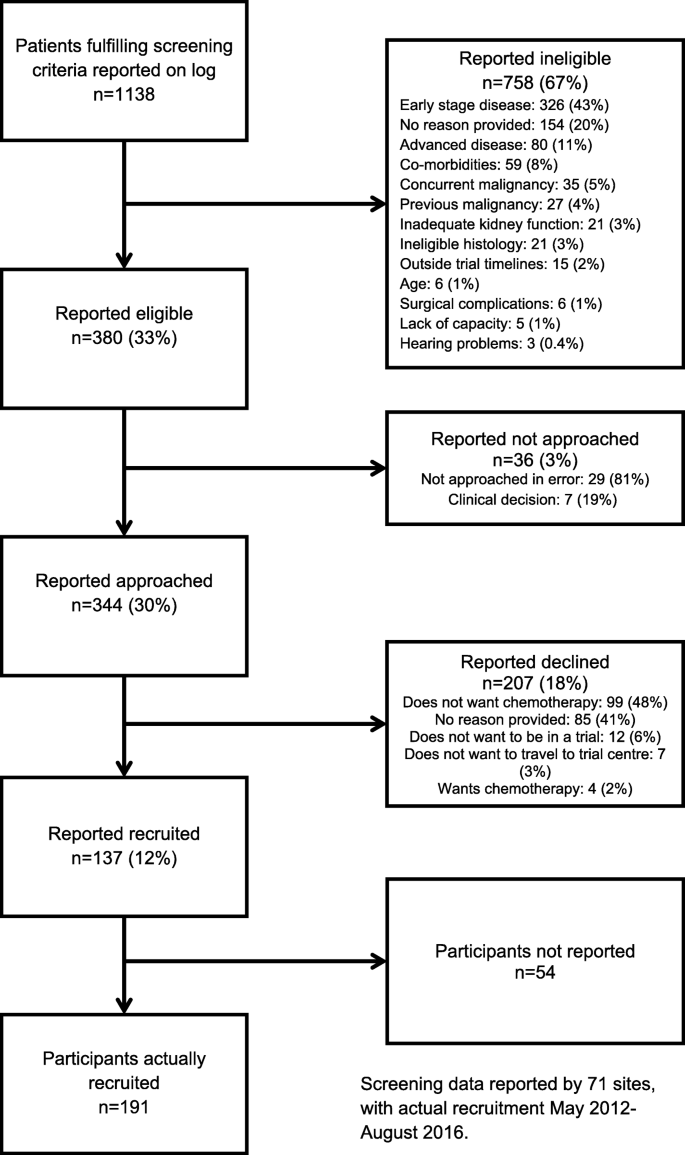
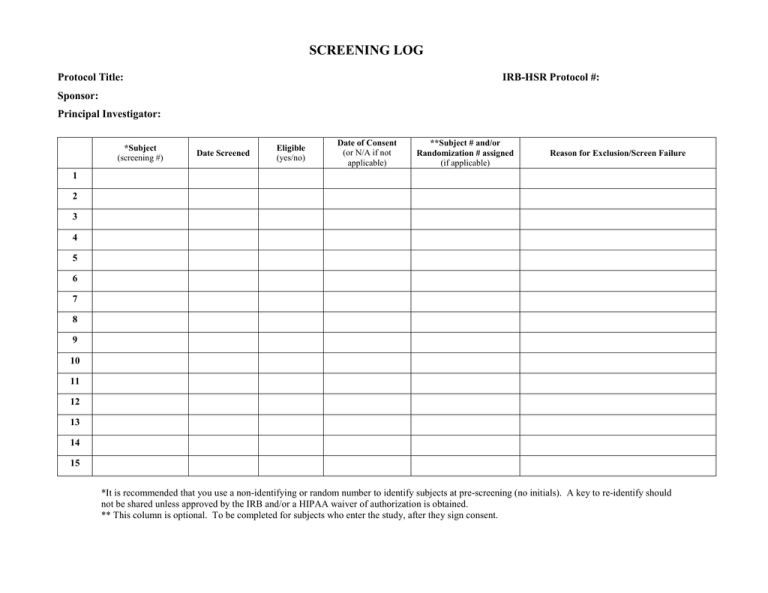
The implementation and utility of patient screening logs in a multicentre randomised controlled oncology trial | Trials | Full Text