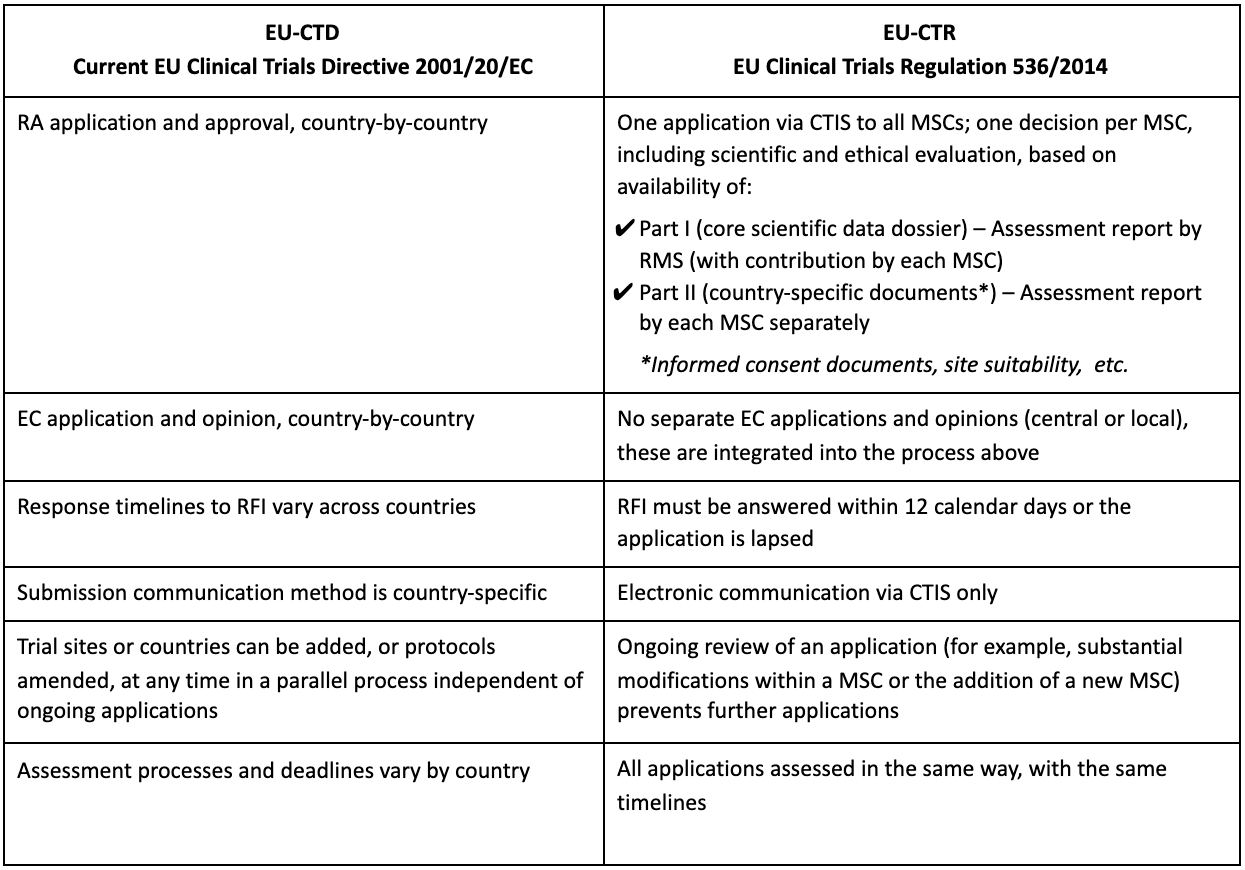
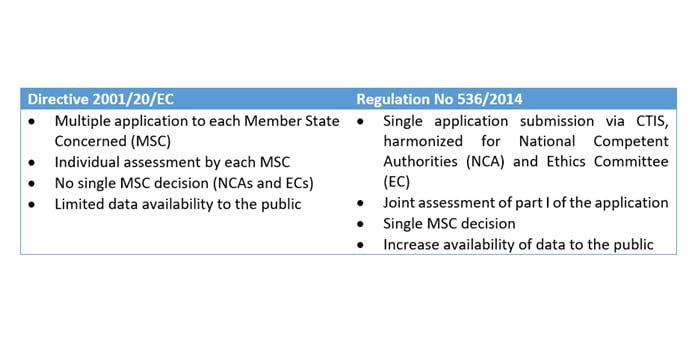
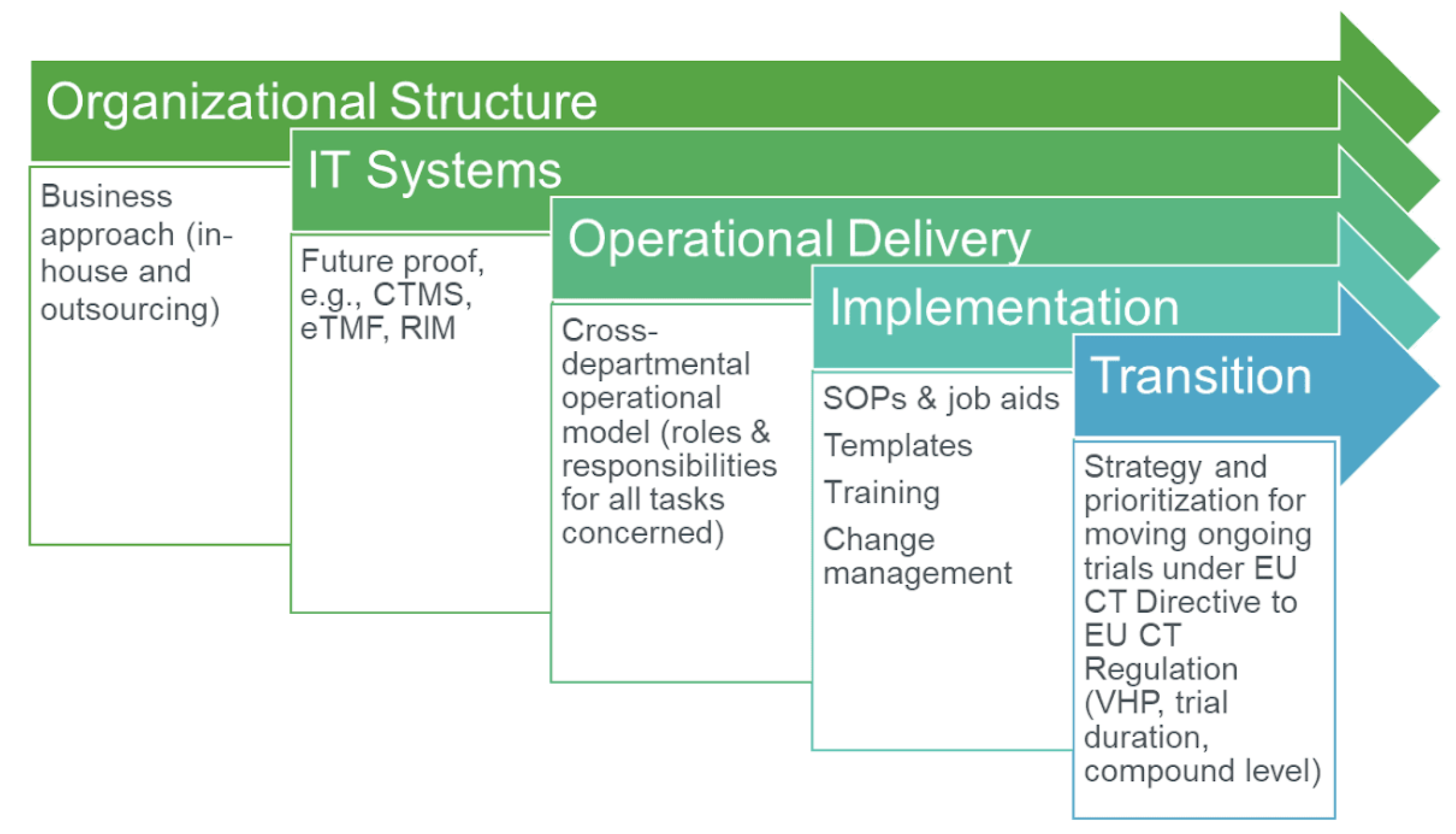
Flow of clinical trials application according to 2001/20/EC Directive.... | Download Scientific Diagram
PDF) Typical investigational medicinal products follow relatively uniform regulations in 10 European Clinical Research Infrastructures Network (ECRIN) countries

EORTC EU Clinical Trials Directives Organisation and Implementation of Cancer Clinical Trials Anastassia Negrouk EORTC Regulatory Affairs Manager Intergroup. - ppt download

Exploring the Impact of the New European Directive on the Pharmaceutical Industry - Clinical Trials Arena

Will the EU Clinical Trials Regulation Support the Innovative Industry in Bringing New Medicines Faster to Patients? | SpringerLink

Timeline impact assessment and Revision of Directive 2001/20/EC (see... | Download Scientific Diagram

White Paper: Pharmaceutical Industry Challenges Facing Clinical Trial Disclosure and Transparency - TrialAssure
EUROPEAN COMMISSION Brussels, 11/04/2012 sanco.ddg1.d.6(2012)501417 VOLUME 10 - G Date of discussion of draft by the ad-hoc gro

PDF) The potential impact of the “Proposal for a Regulation of the European Parliament and of the Council on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC“ on

European Clinical Trial Directive (Directive 2001/20/EC) dr. Cees Smit (NPCF/EGAN) EPF Annual Meeting May 19, Brussels. - ppt download

Directive 2001/20/EC : Clinical trials on medicinal products for human use - Free PDF download | M A N O X B L O G